Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirooka, Shun; Horii, Yuta; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*; Vauchy, R.; Hayashizaki, Kohei; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1313 - 1323, 2023/11
Times Cited Count:3 Percentile:95.99(Nuclear Science & Technology)Additive MOX pellets are fabricated by a conventional dry powder metallurgy method. Nd O
O and Sm
and Sm O
O are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
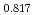 Pu
Pu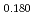 Am
Am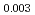 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:4 Percentile:98.08(Materials Science, Multidisciplinary) - and K-montmorillonite
- and K-montmorilloniteKawakita, Ryohei; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Kikuchi, Ryosuke*; Otake, Tsubasa*; Sato, Tsutomu*
Applied Clay Science, 231, p.106722_1 - 106722_7, 2023/01
Times Cited Count:1 Percentile:21.06(Chemistry, Physical)Takahatake, Yoko; Watanabe, So; Irisawa, Keita; Shiwaku, Hideaki; Watanabe, Masayuki
Journal of Nuclear Materials, 556, p.153170_1 - 153170_7, 2021/12
Times Cited Count:1 Percentile:16.35(Materials Science, Multidisciplinary)Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-28-Nendo) (CD-ROM), 1 Pages, 2017/03
no abstracts in English
Nagai, Takayuki; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-27-Nendo) (CD-ROM), 2 Pages, 2016/03
no abstracts in English
Fukuzumi, Masafumi*; Chimi, Yasuhiro; Ishikawa, Norito; Suzuki, Motohiro*; Takagaki, Masafumi*; Mizuki, Junichiro; Ono, Fumihisa*; Neumann, R.*; Iwase, Akihiro*
Nuclear Instruments and Methods in Physics Research B, 245(1), p.161 - 165, 2006/04
Times Cited Count:17 Percentile:74.61(Instruments & Instrumentation)We have performed swift heavy ion irradiations in Fe-50at.%Rh alloys at room temperature. Before and after the irradiations, the magnetic properties and the lattice structure are measured using Superconducting QUantum Interference Device (SQUID) and X-Ray Diffractometer (XRD), respectively. We have also performed X-ray Magnetic Circular Dichroism (XMCD) measurement near the Fe K-edge at the synchrotron radiation facility, SPring-8, to examine the irradiation-induced ferromagnetic state near the specimen surface. We have found that the swift heavy ion irradiations induce the ferromagnetic state in Fe-50at.%Rh alloy below the antiferromagnetism-ferromagnetism transition temperature of the unirradiated alloy and the lattice expasion by 0.3%. For the specimens irradiated with swift heavy ions, we observe the XMCD spectra correponding to ferromagnetisim, which depend on the mass of irradiating ions and/or irradiation fluence. Effects of energy loss through electronic excitation and elastic collisions on lattice and magnetic structures of Fe-Rh alloy are discussed.
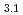 (OCOCH
(OCOCH )
)
 0.9H
0.9H O
OKozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Microporous and Mesoporous Materials, 89(1-3), p.123 - 131, 2006/03
Times Cited Count:11 Percentile:39.34(Chemistry, Applied)Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. Although LTMHSs have recentely attracted attention of researches on anion exchange and intercalation, very limited numbers of reports have been published on their synthesis, characteristics, and applications. This paper describes basic characteristics of a new LTMHS, nickel-copper hydroxide acetate. Hydrothermal Heating of an aqueous solution containing nickel acetate, copper acetate, and hydrogen peroxide to 150 C for 4h yielded a layered compound with an analytical composition of NiCu(OH)
C for 4h yielded a layered compound with an analytical composition of NiCu(OH)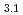 (OCOCH
(OCOCH )
) 0.9H
0.9H O. This compound does not take up Cl
O. This compound does not take up Cl and NO
and NO
 in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
 and AsO
and AsO
 . This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl
. This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl and NO
and NO
 .
.
Kozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Journal of Materials Research, 20(11), p.2997 - 3003, 2005/11
Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. LTMHSs have lately attraced attention of researchs on anion exchange and intercalation but very limited numbers of reports have been published on their synthesis, characteristics, and application. This study reports basic properties of a layered copper hyroxide acetate synthesized by a method modified from that of the previous studies. Titration of copper acetate solution with a dilute NaOH solution to pH 6.5 and subsequent aging at 313 K yielded a layered copper hydroxide acetate. This compound has some properties similar to those of the previously known copper hydroxide acetate, Cu (OH)
(OH) (OCOCH
(OCOCH )H
)H O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
Inoue, Yutaka; Kobayashi, Kazuhiro; Yamaki, Tetsuya; Asano, Masaharu; Kubota, Hitoshi*; Yoshida, Masaru
Dai-2-Kai 21-Seiki Rengo Shimpojiumu; Kagaku Gijutsu To Ningen Rombunshu, p.257 - 260, 2003/00
We prepared crosslinked fluoropolymer electrolyte membranes for use in fuel cells and then investigated their structural properties by X-ray diffraction (XRD) analysis. The radiation-induced grafting of styrene into crosslinked polytetrafluoroethylene (PTFE) films and subsequent sulfonation enabled us to obtain the electrolyte membrane with a sufficient ion exchange capacity, which exceeds that of the commercially-available film, Nafion. As the crosslinking and styrene grafting reactions proceeded, the size of the PTFE crystallites in the film became smaller, thereby decreasing the film crystallinity. Interestingly, in contrast to Nafion, the resulting sulfonated membranes were found to have high crystallinity.
Kawamura, Hiroyuki; Takahashi, Masamitsu; Mizuki, Junichiro
Journal of the Electrochemical Society, 149(11), p.C586 - C591, 2002/11
Times Cited Count:14 Percentile:44.55(Electrochemistry)no abstracts in English
Dai, S.*; Hu, L.*; Sugiyama, Akira; Izawa, Yasukazu*; Liu, Z.*; Jiang, Z.*
Chinese Science Bulletin, 47(3), p.255 - 259, 2002/02
Times Cited Count:7 Percentile:51.71(Multidisciplinary Sciences)A new type of ytterbium doped phosphate laser glass has been produced and studied about physical and optical properties such as thermo-mechanical properties, nonlinear properties, crystalline phase, micro defects and spectroscopic properties. In the glass forming process, newly developed OH removing technology was adopted. From the reduction of OH concentration in the glass, upper state fluorescence lifetime reached 2.2 ms. Additionally, the glass shows remarkable athermal property of 0.42 10E
10E /K, which is around one-tenth of QX/Yb glass.
/K, which is around one-tenth of QX/Yb glass.
Kawamura, Hiroyuki; Takahashi, Masamitsu; Hojo, Nobuhiko*; Miyake, Masao*; Murase, Kuniaki*; Tamura, Kazuhisa*; Uosaki, Kohei*; Awakura, Yasuhiro*; Mizuki, Junichiro; Matsubara, Eiichiro*
Journal of the Electrochemical Society, 149(2), p.C83 - C88, 2002/02
Times Cited Count:6 Percentile:21.78(Electrochemistry)The structure of a Te layer formed on a Au(111) substrate by underpotential deposition (UPD) in an electrolytic solution has been studied using in-situ surface X-ray diffraction technique. The measurements were carried out for a series of samples which were kept at UPD potential for 4 to 59 hours. The results revealed that the Te UPD layer is unstable. The top layer is analyzed to consist of the UPD Te atoms and Au atoms which diffuse from the Au(111) substrate. Also, the Te UPD layer does not have the structure with periodicity reported in previous works, such as (

 ) R30
) R30 after ample time elapses. Stripping voltammetry for the Te UPD layer shows that the interaction between Te and Au increases with time, supporting the finding that the top layer is a mixture of Te and Au.
after ample time elapses. Stripping voltammetry for the Te UPD layer shows that the interaction between Te and Au increases with time, supporting the finding that the top layer is a mixture of Te and Au.
 films by pulsed laser deposition technique
films by pulsed laser deposition techniqueYamamoto, Shunya; Sumita, Taishi; Sugiharuto; Miyashita, Atsumi; Naramoto, Hiroshi
Thin Solid Films, 401(1-2), p.88 - 93, 2001/12
Times Cited Count:129 Percentile:96.77(Materials Science, Multidisciplinary)The epitaxial growth of high-quality TiO films has attracted much interest from the viewpoints of basic science and applications. In this study, it is shown that TiO
films has attracted much interest from the viewpoints of basic science and applications. In this study, it is shown that TiO films with anatase and rutile structure can be prepared by pulsed laser deposition (PLD) with a Nd-YAG laser under the controlled O
films with anatase and rutile structure can be prepared by pulsed laser deposition (PLD) with a Nd-YAG laser under the controlled O atmosphere. The TiO
atmosphere. The TiO films with 200 nm thickness were prepared on the SrTiO
films with 200 nm thickness were prepared on the SrTiO , LaAlO
, LaAlO , LSAT and
, LSAT and  -Al
-Al O
O single crystal substrates. The epitaxial films were analyzed by Rutherford backscattering spectrometry (RBS) combined with channeling and X-ray diffraction for the crystallographic relationships with the substrates. The high quality anatase TiO
single crystal substrates. The epitaxial films were analyzed by Rutherford backscattering spectrometry (RBS) combined with channeling and X-ray diffraction for the crystallographic relationships with the substrates. The high quality anatase TiO (001) films have been successfully prepared on the LaAlO
(001) films have been successfully prepared on the LaAlO (001), LSAT (001) and SrTiO
(001), LSAT (001) and SrTiO (001) substrates about 500
(001) substrates about 500 C substrate temperature. Also the high quality rutile TiO
C substrate temperature. Also the high quality rutile TiO (100) and TiO
(100) and TiO (001) films were obtained on the
(001) films were obtained on the  -Al
-Al O
O (0001) and (10
(0001) and (10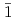 0), respectively.
0), respectively.
Yaita, Tsuyoshi; Okamoto, Yoshihiro
Hoshako, 14(1), p.42 - 51, 2001/02
no abstracts in English
Yaita, Tsuyoshi
Proceedings of International Youth Nuclear Congress 2000 (CD-ROM), 4 Pages, 2000/00
no abstracts in English
Wu, Z. P.*; Miyashita, Atsumi; Yamamoto, Shunya; Abe, Hiroaki; Nashiyama, Isamu; Narumi, Kazumasa; Naramoto, Hiroshi
Journal of Applied Physics, 86(9), p.5311 - 5313, 1999/00
Times Cited Count:59 Percentile:88.49(Physics, Applied)no abstracts in English
 crystals with RBS/PIXE/XRD
crystals with RBS/PIXE/XRDR.Q.Zhang*; Yamamoto, Shunya; Dai, Z.*; Narumi, Kazumasa; Aoki, Yasushi*; Naramoto, Hiroshi; Miyashita, Atsumi
International Journal of PIXE, 7(3&4), p.265 - 275, 1997/00
no abstracts in English
 solid solution
solid solutionSerizawa, Hiroyuki; Shiratori, Tetsuo; Fukuda, Kosaku; Fujino, Takeo*; Sato, Nobuaki*
Journal of Alloys and Compounds, 218, p.149 - 156, 1995/00
Times Cited Count:3 Percentile:37.09(Chemistry, Physical)no abstracts in English
Kamei, Gento; Yusa, Yasuhisa; Sasaki, Noriaki
PNC TN8410 91-253, 8 Pages, 1992/01
Time-temperature conditions and water chemistry on illitization at the Murakami deposit in central Japan were determined. The extent of the illitization and time-temperature condition estimated were as follows: the duration for conversion from 0 to 80% illite (volumetric ratio) was approximately 3.5 Ma in the temperature range from 340 to 100 C, Conversion from 0 to 40% requires approximately 3.0 Ma in the temperature range from 240 to 100
C, Conversion from 0 to 40% requires approximately 3.0 Ma in the temperature range from 240 to 100 C, During 2.0Ma in the temperature range from 160 to 100
C, During 2.0Ma in the temperature range from 160 to 100 C, however, illite was scaroely observed, Water chemistry is estimated from two approaches, namely laboratory experiment and numerical analysis. The former is an interactive experiment between seawater and the tuff of the deposit. The latter is a calculation based on the difference of bulk composition between illitized and non-illitized tuff. The extent of each ionic concentration is inferred to be as follows: K
C, however, illite was scaroely observed, Water chemistry is estimated from two approaches, namely laboratory experiment and numerical analysis. The former is an interactive experiment between seawater and the tuff of the deposit. The latter is a calculation based on the difference of bulk composition between illitized and non-illitized tuff. The extent of each ionic concentration is inferred to be as follows: K ; 560 to 6400, Mg
; 560 to 6400, Mg ; 800 to 1700, Ca
; 800 to 1700, Ca ; 360 to 2900, Na
; 360 to 2900, Na ; 9400 to 15000 (mg/l).
; 9400 to 15000 (mg/l).